By Mireia Larrosa Godall, PhD student, Alphey Lab, University of York
From October 18-22, I attended the 2023 annual meeting of the American Society of Tropical Medicine and Hygiene (ASTMH) in Chicago. This annual conference, held over the course of five days, gathered participants from around the world in the fields of tropical medicine and global health. As part of the meeting, I had the opportunity to present a poster on findings from our research at the University of York to develop new tools to control the invasive malaria mosquito Anopheles stephensi.
An. stephensi is an urban malaria vector mainly present in South Asia and the Arabian Peninsula. Since 2012 it has been detected on the African continent where it has rapidly spread, posing a significant threat to malaria control efforts in Africa. An. stephensi thrives in man-made environments and has been found to be resistant to several insecticides, making the development of novel approaches to control this vector crucial. With the predicted increase in the proportion of urban population and recent evidence of An. stephensi driving an urban malaria outbreak in Africa, it will be important to control it before it becomes endemic.
Our research primarily focuses on CRISPR/Cas9-based gene drives, a potential new tool to control mosquito populations. CRISPR/Cas9-based gene drives allow scientists to precisely insert genetic sequences into genomes. These inserted genetic sequences are able to cut a specific target sequence in the mosquitoes’ genome, triggering a DNA repair mechanism. When the DNA is repaired by homologous recombination (also known as homing), the organism ends up with two copies of the inserted genetic sequence, rather than the initial one. This allows the drive to be transmitted to up to 100% of its offspring, rather than the 50% for a ‘normally’ inherited gene in that situation. This biased inheritance allows the modification to spread through the target population.
However, any method of mosquito population control – including gene drives – is prone to resistance. Changes occurring in the target sequence of wild-type mosquitoes could stop the spread of the gene drive into the mosquito population. Part of our work therefore consists of exploring strategies that could help overcome resistance to gene drive systems. The poster I presented specifically sheds light on the multiplexing strategy as a potential tool to overcome resistance to gene drives in An. stephensi mosquitoes.
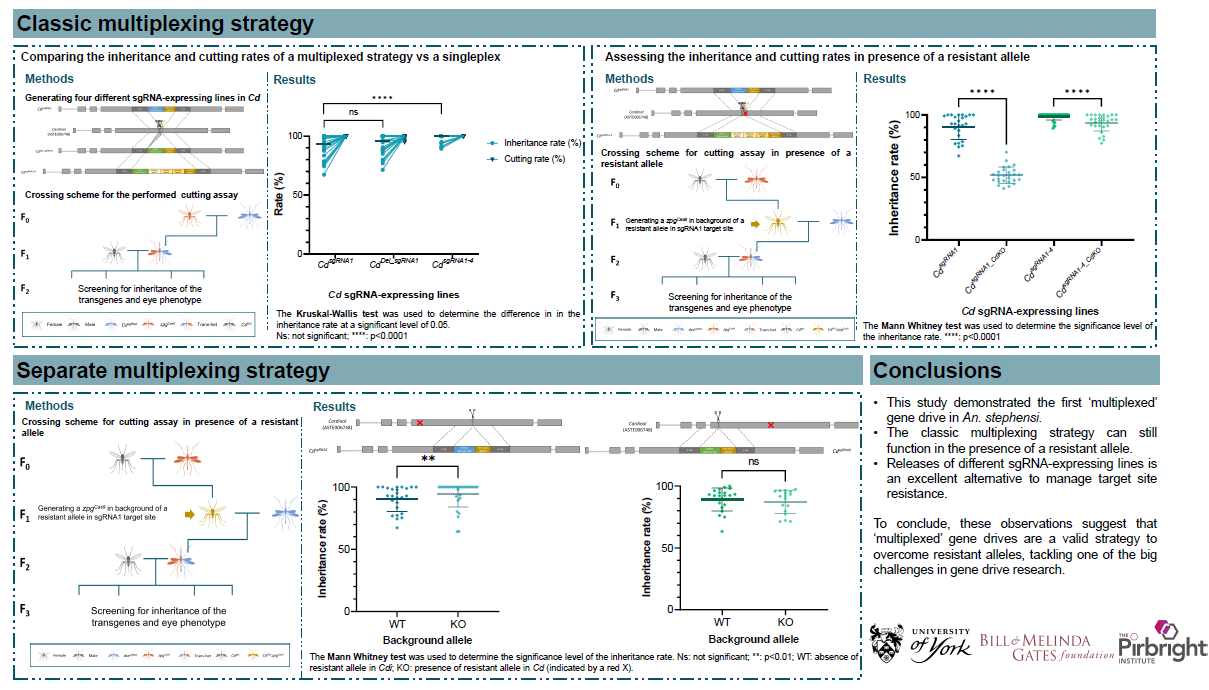 “Assessing two multiplexing strategies in Anopheles stephensi” poster. Image: Mireia Larrosa Godall.
“Assessing two multiplexing strategies in Anopheles stephensi” poster. Image: Mireia Larrosa Godall.
What is a multiplexing strategy? Multiplexing strategies allow us to target multiple sites of the genome rather than a single one, helping the gene drive to overcome resistance occurring in one of the target sequences. Our research aimed to explore two forms of such strategies, and how these could help us reduce the likelihood of resistance persisting. Our findings underscored the promising potential of multiplexing to overcome resistance whilst maintaining high levels of homing.
As we move forward in the fight against malaria, it becomes increasingly evident that we need to embrace innovative tools to stay one step ahead of emerging challenges. At this year’s ASTMH Annual meeting, I particularly enjoyed learning more about the many promising solutions being explored to navigate the ever-evolving landscape of disease control and improve global health.
Recent posts
